ASOs usually consist of 15-20 nucleotides that are complementary to the target mRNA sequence. Chemically modified ribonucleotides are used to protect against nuclease degradation, improve target affinity and delivery to the intended target/tissue/organ.
In the FDA’s Guidance, individualized ASOs are limited to well-characterized antisense chemical classes where substantial nonclinical information and clinical experience are available. These include single-stranded phosphorothioate or mixed phosphorothioate/phosphodiester with or without 2-methoxyethyl substituted oligonucleotides (by systemic or intrathecal route), and phosphorodiamidate morpholino oligonucleotides (by systemic route).
Oligonucleotide phosphorothioate modification is the most widely used backbone variant: one of the oxygen atoms in the phosphate backbone is replaced by sulfur, providing nuclease resistance as well as facilitating cell membrane entry.
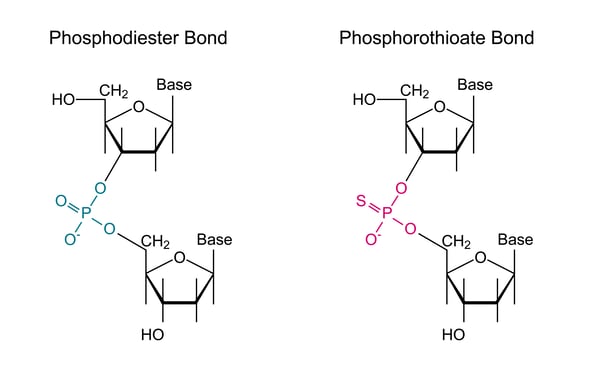
However, phosphorothioates show sequence-independent, but length-dependent binding to cellular proteins (heparin-binding molecules) and show up as phosphorothioate-linked thrombocytopenia (low blood platelet count).
Increasing the number of phosphorothioate bonds in an oligo tends to lower the melting temperature (Tm) by around 0.5 °C per PS bond, thereby affecting hybridisation to the target. Introduction of too many sulfurization process during synthesis creates stereogenic α-phosphorus atoms, giving rise to diastereomers. These stereoisomers have different functional properties that can limit antisense pairing.
For intrathecal dosing, RNA 2′-O-methylation is the only remaining RNA modification permitted by the FDA for use in individualized ASOs. RNA 2′-O-methylation is highly abundant in noncoding RNAs and occurs in the 5′ cap of virtually all messenger RNAs (mRNAs) in higher eukaryotes.
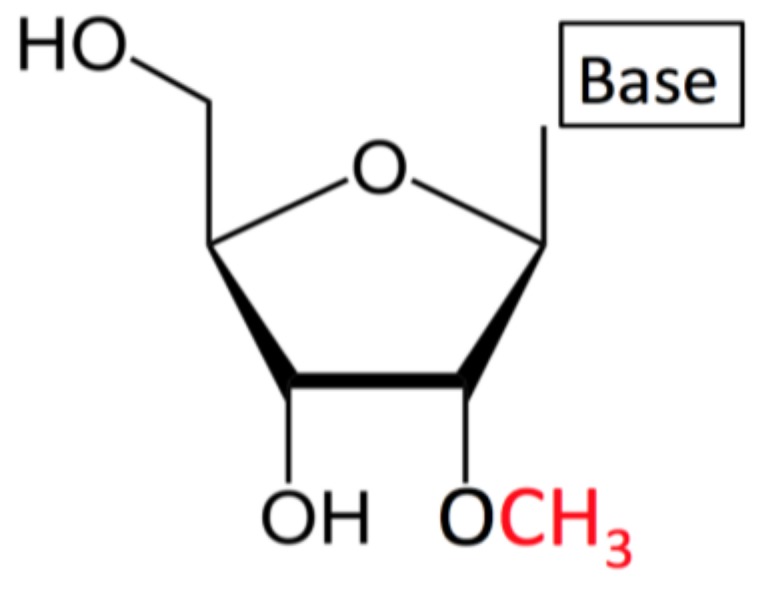
2′-O-methylation has the potential to affect RNAs in multiple ways as it can increase their hydrophobicity, protect them from nuclease attacks, stabilize helical structures, and affect their interactions with proteins or other RNAs.